Henderson Laboratory
Protein Pathologies and Genetic Risk in Neurodegeneration
Parkinson’s disease (PD) and dementia with Lewy bodies (DLB) are progressive neurodegenerative diseases affecting movement and cognitive function in patients. These two diseases share a common pathology — aggregates bearing misfolded forms of the normally synaptic protein α-synuclein. While these diseases begin gradually, they progress relentlessly, and this progression of symptoms is associated with the accumulation of misfolded α-synuclein in more and more regions of the brain and the misfolding of additional proteins, including tau.
PD and DLB bear an additional commonality — the lack of disease-modifying treatments. Substantial evidence suggests that either disrupting the formation of protein pathologies or the transmission of pathology through the brain will result in stabilization, and perhaps improvement, of symptoms. Susceptibility to these diseases depends on several factors, including genetic background. Mutations in proteins like leucine-rich repeat kinase 2 (LRRK2) and glucocerebrosidase can predispose individuals to developing PD or DLB and targeting these proteins may provide therapeutic benefit for patients as well.
The Henderson Laboratory takes a two-pronged approach to addressing neurodegenerative disease. One approach is to understand what goes wrong in neurons and the brain to lead to neurodegeneration by probing disrupted cellular pathways, mapping how pathology spreads through the brain and investigating the impact of genetic risk factors on disease progression. The second approach is to use what we learn about these diseases to develop and evaluate potential therapeutic treatments. The laboratory utilizes primary neuron cultures to investigate cellular pathways disrupted in disease and screen therapeutic molecules. We also use animal models of disease to understand how pathological proteins spread through the brain and to evaluate the likely efficacy of top therapeutic candidates. It is the mission of the Henderson Laboratory to use rigorous scientific methods to understand neurodegenerative diseases and to leverage that knowledge to develop and evaluate treatments for these devastating diseases.
News
Learn More
Not just a harmless accessory, tau’s ‘fuzzy coat’ contributes to Alzheimer’s progression

University of Minnesota, Van Andel Institute receive two-year, $5.8M grant to study Parkinson’s disease and cellular senescence

How understanding proteins can inform new Parkinson’s treatments
Our Impact
We’re raising thousands to save millions.
We’re turning hope into action for the millions of people around the world affected by diseases like cancer and Parkinson’s. Find out how you can help us make a difference.
- 122 peer-reviewed papers published in 2024, 63 of which were in high-impact journals
- 15 VAI-SU2C Epigenetics Dream Team clinical trials launched to date
- 10 clinical trials co-funded by VAI & Cure Parkinson's (out of 41 total International Linked Clinical Trials Program trials)
Michael Henderson, Ph.D.
Associate Professor, Department of Neurodegenerative Science; Director, VAI Brain Bank
Areas of Expertise
Parkinson’s disease (PD), Alzheimer’s disease (AD), neurodegenerative disease models, pathological protein cell-to-cell transmission, GBA1, α-synuclein, tau
Biography
Michael Henderson, Ph.D., is an associate professor in the Department of Neurodegenerative Science at Van Andel Institute and the founding director of the VAI Brain Bank. His research centers on understanding why certain neurons and brain circuits are especially vulnerable to neurodegenerative diseases such as Parkinson’s disease, dementia with Lewy bodies and Alzheimer’s disease.
Dr. Henderson uses an integrative approach that spans molecular and structural neuropathology, spatial transcriptomics, advanced microscopy and in vivo modeling. His work has revealed how pathogenic proteins including α-synuclein and tau propagate through brain networks, how genetic and cellular factors influence selective vulnerability, and how co-pathologies interact to shape disease progression. These efforts aim to define the biological rules that govern neurodegeneration and to identify new entry points for therapeutic intervention.
Dr. Henderson earned his Ph.D. in neuroscience from Yale University and completed postdoctoral training at the University of Pennsylvania, where he developed expertise in neurodegenerative disease mechanisms and neuropathology. In addition to the lab, Dr. Henderson directs the VAI Brain Bank, a resource that enables discoveries across the Institute and the broader scientific community. He is also an active educator, serving as a course director and mentor within Van Andel Institute Graduate School.
Selected Publications
Authors marked with an asterisk contributed equally.
2025
Soto-Avellaneda A, Prigent A, Meyerdirk L, Schautz N, Pospisilik JA, Brundin L, Henderson MX. 2025. Helicobacter pylori infection and a-synuclein pathology drive parallel neurodegenerative pathways in the substantia nigra. J Neuroinflammation 22(1).
Kasen A, Lövestam S, Breton L, Meyerdirk L, McPhail JA, Piche K, Louwrier A, Capan CD, Lee H, Scheres SH, Henderson MX. 2025. Seed structure and phosphorylation in the fuzzy coat impact tau seeding competency. Nat Commun 16(1).
Dues DJ, Erb ML, Kasen A, Vatsa N, Williams ET, Nguyen APT, Henderson MX, Moore DJ. 2025. Pathological α–synuclein elicits granulovacuolar degeneration independent of tau. Transl Neurodegener 14(1):31.
2024
Drougard A, Ma EH, Wegert V, Sheldon R, Panzeri I, Vatsa N, Apostle S, Facnocchi L, Schaf J, Gossens K, Völker J, Pang S, Bremser A, Dror E, Giacona F, Sagar, Henderson MX, Prinz M, Jones RG, Pospisilik JA. 2024. An acute microglial metabolic response controls metabolism and improves memory. eLife.
Geertsma HM, Fisk ZA, Sauline L, Prigent A, Kurgat K, Callaghan SM, aSCENT-PD Consortium, Henderson MX#, Rousseaux MWC#. 2024. A topographical atlas of α-synuclein dosage and cell type-specific expression in adult mouse brain and peripheral organs. npj Parkinson’s 10(65).
# Co-corresponding authors
Lubben N, Byrnildsen JK, Webb CM, Li HL, Leyns CEG, Changolkar L, Zhang B, Meymand ES, O’Reilly M, Madaj Z, DeWeerd D, Fell MJ, Lee VYM, Bassett DS, Henderson MX. 2024. LRRK2 kinase inhibition reverses G2019s mutation-dependent effects on tau pathology progression. Transl Neurodegener 13(1):13.
Goralski TM, Meyerdirk L, Breton L, Brasseur L, Kurgat K, DeWeerd D, Turner L, Becker K, Adams M, Newhouse DJ, Henderson MX. 2024. Spatial transcriptomics reveals molecular dysfunction associated with cortical Lewy pathology. Nat Commun 15:2642.
2023
Leyns CG, Prigent A, Beezhold B, Yao L, Hatcher NG, Tao P, Kang J, Suh E, Van Deerlin VM, Trojanowski JQ, Lee VMY, Kennedy ME, Fell MJ, Henderson MX. 2023. Glucocerebrosidase activity and lipid levels are related to protein pathologies in Parkinson’s disease. NPJ Parkinson’s Dis 9(1):74.
Peelaerts W, Mercado G, George S, Villumsen M, Kasen A, Aguileta, Linstow C, Sutter AB, Kuhn E, Stetzik L, Sheridan R, Bergkvist L, Meyerdirk L, Lindqvist A, Escobar Galvis ML, Van den Haute C, Hultgren SJ, Baekelandt V, Pospisilik JA, Brudek T, Aznar S, Steiner JA, Henderson MX, Brundin L, Ivanova MI, Hannan TJ, Brundin P. 2023. Urinary tract infections trigger synucleinopathy via the innate immune response. Acta Neuropathol 6.
2022
Chen L, Nagaraja C, Daniels S, Fisk ZA, Dvorak R, Meyerdirk L, Steiner JA, Escobar Galvis ML, Henderson MX, Rousseaux MWC, Brundin P, Chu HY. 2022. Synaptic location is a determinant of the detrimental effects of α-synuclein pathology to glutamatergic transmission in the basolateral amygdala. eLife 11:e78055.
Henderson MX, Henrich MT, Geibl FF, Oertel WH, Brundin P, Surmeier DJ. 2022. The roles of connectivity and neuronal phenotype in determining the pattern of α-synuclein pathology in Parkinson’s disease. Neurobiol Dis 168:105687.
Stetzik L, Mercado G, Smith L, George S, Quansah E, Luda K, Schulz E, Meyerdirk L, Lindquist A, Bergsma A, Jones RG, Brundin L, Henderson MX, Pospisilik JA, Brundin P. 2022. A novel automated morphological analysis of IBA1+ microglia using a deep learning assisted model. Front Cell Neurosci 16:944875.
2021
Cornblath EJ, Li HL, Changolkar L, Zhang B, Brown HJ, Gathagan RJ, Olufemi MF, Trojanowski JQ, Bassett DS, Lee VYM, Henderson MX. 2021. Computational modeling of tau pathology spread reveals patterns of regional vulnerability and the impact of a genetic risk factor. Sci Adv 7(24):eabg6677.
Henderson MX, Changolkar L, Trojanowski JQ, Lee VMY. In press. LRRK2 kinase activity does not alter cell-autonomous tau pathology development in primary neurons. J Parkinson’s Dis.
2020
Henderson MX, Sedor S, McGeary I, Cornblath EJ, Peng C, Riddle DM, Li HL, Zhang B, Brown H, Olufemi MF, Bassett DS, Trojanowski JQ, Lee VMY. 2020. Glucocerebrosidase activity modulates neuronal vulnerability to α-synuclein pathology. Neuron 105:1–15.
Henderson MX, Covell DJ, Chung CHY, Pitkin RM, Sandler RM, Decker SC, Riddle DM, Zhang B, Gathagan RJ, James MJ, Trojanowski JQ, Brunden K, Lee VMY, Luk K. 2020. Characterization of novel conformation-selective α-synuclein antibodies as potential immunotherapeutic agents for Parkinson’s disease. Neurobiol Dis 136:104712.
2019
Henderson MX, Sengupta M, Trojanowski JQ, Lee VMY. 2019. Alzheimer’s disease tau is a prominent pathology in LRRK2 Parkinson’s disease. Acta Neuropathol Commun 7(1):183.
Henderson MX, Cornblath EJ, Darwich A, Zhang B, Brown H, Gathagan H, Sandler R, Bassett DS, Trojanowski JQ, Lee VMY. 2019. Spread of α-synuclein pathology through the brain connectome is modulated by selective vulnerability and predicted by network analysis. Nat Neuro 22:1248–1257.
Reviewed in:
Kuhl, E. 2019. Connectomics of neurodegeneration. Nat Neuro 22:1200–1202.
Lempriere S. 2019. Connectivity and vulnerability determine α-synuclein spread. Nat Rev Neurol.
Henderson MX, Trojanowski JQ, Lee VMY. 2019. α-Synuclein pathology in Parkinson’s disease and related α-synucleinopathies. Neurosci Lett 709:134316.
Henderson MX, Sengupta M, McGeary I, Zhang B, Olufemi MF, Brown H, Trojanowski, JQ, Lee VMY. 2019. LRRK2 inhibition does not impart protection from α-synuclein pathology and neuron death in non-transgenic animals. Acta Neuropathol Commun 7:28.
2018
Henderson MX, Peng C, Trojanowski, JQ, Lee VMY. 2018. LRRK2 activity does not dramatically alter α-synuclein pathology in primary neurons. Acta Neuropathol Commun 6:45.
2017
Henderson MX, Chung CH-Y, Riddle DM, Zhang B, Gathagan RJ, Seeholzer SH, Trojanowski, JQ, Lee VMY. 2017. Unbiased proteomics of early Lewy body formation model implicates active microtubule affinity-regulating kinases (MARKs) in synucleinopathies. J Neurosci 37(24):5870–5884.
2016
Henderson MX*, Wirak GS*, Zhang YQ, Dai F, Ginsberg SD, Dolzhanskaya N, Staropoli JF, Nijssen PC, Lam TT, Roth AF, Davis NG, Dawson G, Velinov M, Chandra SS. 2016. Neuronal ceroid lipofuscinosis with DNAJC5/CSPα mutation has PPT1 pathology and exhibit aberrant protein palmitoylation. Acta Neuropathol 131(4):621–637.
2012
Zhang Y*, Henderson MX*, Colangelo CM, Ginsberg S, Bruce C, Wu T, Chandra SC. 2012. Identification of CSPα clients reveals a role in dynamin 1 regulation. Neuron 74:136–150.
Reviewed in: Sheng J, Wu L. 2012. Cysteine string protein α: A new role in vesicle recycling. Neuron 74:6–8.


Libby Breton, M.S.
Senior Research Technician, Department of Neurodegenerative Science

Jordan Cook
Laboratory Aide, Department of Neurodegenerative Science

Ashley Douglass, B.S.
Senior Administrative Assistant II

Kevin Kurgat
Research Technician, Department of Neurodegenerative Science


Lindsay Meyerdirk, M.S.
Lab Manager, Department of Neurodegenerative Science


Sydney Shenk
Assistant Research Technician, Department of Neurodegenerative Science


Naman Vatsa, Ph.D.
Postdoctoral Fellow, Henderson Laboratory
Parkinson's disease

Parkinson’s and Alzheimer’s are progressive, life-altering diseases that affect movement and cognitive function in individuals. The genes we are born with and the environment we grow up in influence our likelihood of developing disease but, in most cases, the cause of disease is unknown. What we know is that these diseases are characterized by the misfolding of normal brain proteins into aggregated forms that are toxic to cells. The Henderson Lab focuses on these aggregates, trying to understand how and why they form, and leveraging that knowledge to inform potential therapeutic interventions.
Some of the main questions we are currently addressing are:
Which cells are vulnerable to developing protein pathologies?
Aging is the major risk factor for all major neurodegenerative diseases. But not all of us develop disease. Even within a diseased brain, not all neurons are affected. What makes specific neuron types vulnerable? In addition to looking at single pathologies, we are leveraging brains and models with multiple pathologies (α-synuclein, tau) to understand how specific molecular programs determine how neurons develop pathology. We recently found that α-synuclein and tau almost always aggregate in different neurons, even when they are in the same brain, implicating selective cell type vulnerability.
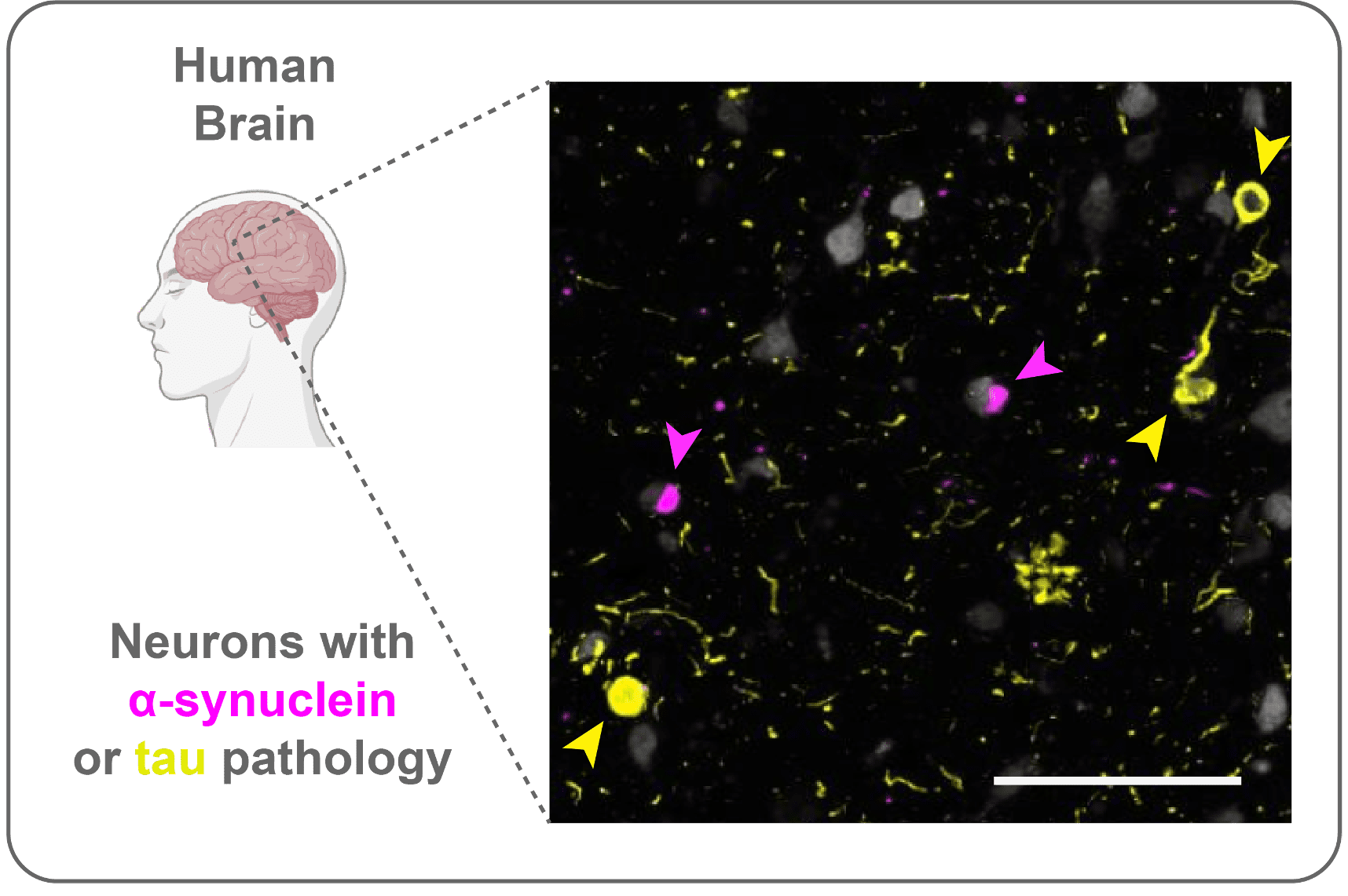
How do neurons respond to protein aggregation?
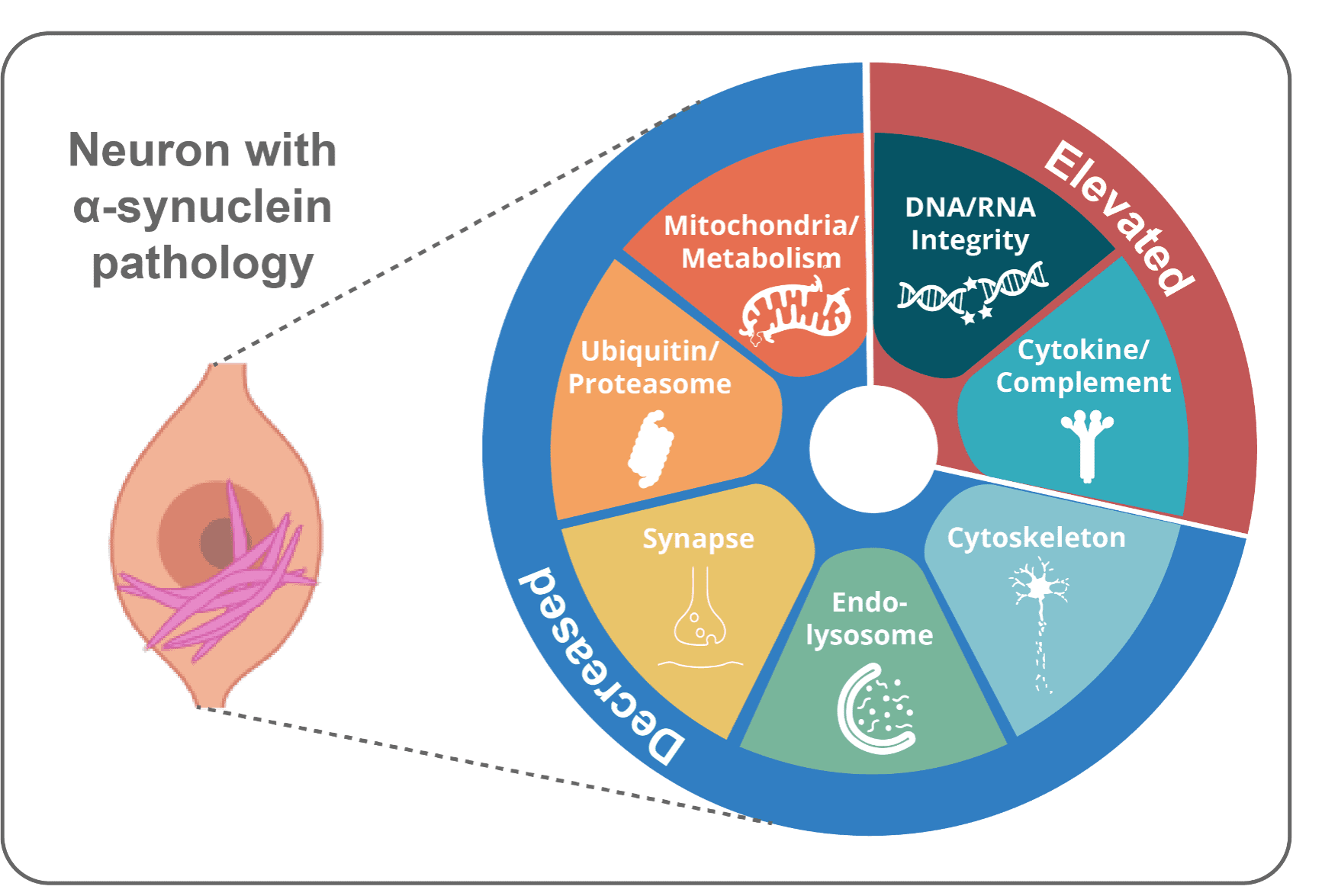
One would think badly. No cell wants clumps of misfolded proteins and dysfunctional organelles clogging up their machinery. But they do the best they can, and neurons may survive years with aggregates. We recently identified a Lewy-Associated Molecular Dysfunction from Aggregates (LAMDA) signature associated with α-synuclein aggregates. This involves downregulation of homeostatic (mitochondria, synapse maintenance) pathways and upregulation of stress pathways (DNA damage repair, complement/cytokine). We aim to improve that cellular response, clear aggregates and improve neuronal function.
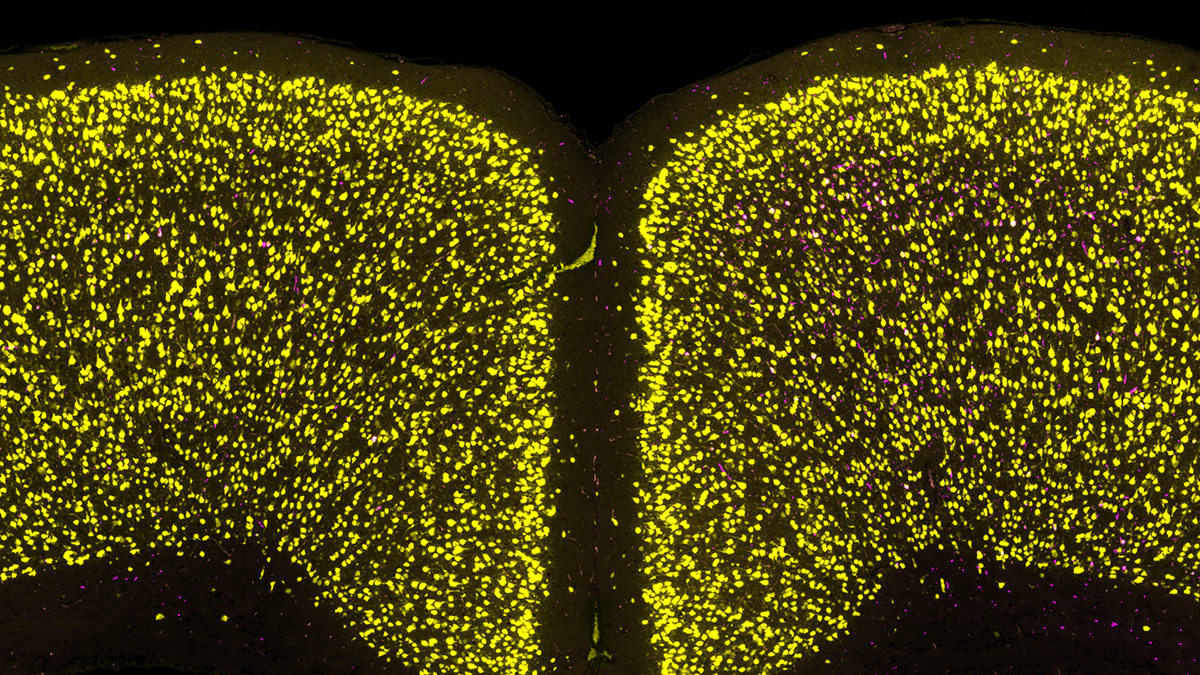
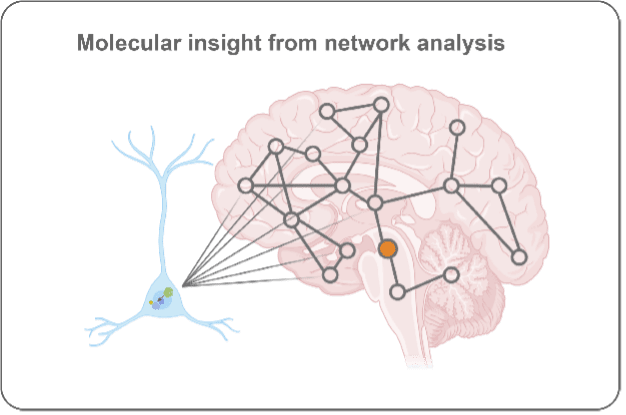
What brain region and network factors control pathology progression through the brain?
Unfortunately, symptoms for Parkinson’s and Alzheimer’s diseases are progressive. This insidious worsening of symptoms is tied to pathology becoming more extensively spread throughout the brain. We leverage brain mapping and network analysis to determine how pathology progresses through the brain, what underlies regional vulnerability to pathology, and what factors (genetic, environmental, molecular) influence pathology progression.
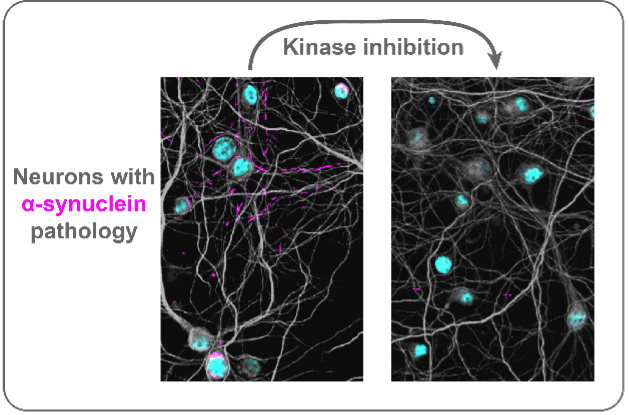
Can we leverage our knowledge to develop disease-modifying therapeutics?
A major goal for the lab is to turn our knowledge of disease into treatments. Our recent network analysis of pathology progression led us to molecular targets underlying vulnerability to developing pathology. From this, we identified a group of kinases that appear involved in aggregate formation and clearance. Inhibit those kinases and we reverse pathology and rescue neurons. We are now pushing to see if these kinases could be new therapeutic targets for Parkinson’s disease.